
At Intrinsic, we’ve been implementing Clinical Trial Management Systems (CTMSs) and have been part of the eClinical evolution since Part 11 was drafted back in 1997. During that time, we’ve had to answer a lot of questions, starting with, ‘Do I have to validate my CTMS?’. Over the years that question was replaced with, ‘Do I really need a CTMS?’. Now, believe it or not, the question that I hear more and more frequently, is ‘What is a CTMS?’. What is a clinical trial management system used for these days?
When I was first asked, ‘What is a CTMS?’, I admit I was a bit taken back. It wasn’t until one of my clients asked me to come in to discuss this topic with their executive leadership team that I understood why there was so much confusion over what I thought to be a straightforward question. The reason everyone was confused about CTMS software was because they all had different interactions with their ‘CTMS’, which turned out not to be a single CTMS system. In fact, none of the executives had ever used their actual CTMS, rather most of them were referring to clinical trial reports that were sent to them or viewed somewhere in their ‘CTMS Portal’, which was actually a SharePoint reporting site, not a CTMS system. Furthermore, the CTMS reports contained data from multiple systems, including CTMS, EDC, IVRS, Microsoft Project, their contracts database, and information manually reported such as status updates, issues, and risks associated with each study and program. In addition, their IT department had recently brought in several vendors to demo each of their CTMS systems, but instead they all showed off their entire integrated suite of eClinical systems. All of this together had caused the confusion, as there was no clear distinction between one CTMS system and the other CTMS system.
What is a CTMS?
Once I realized why there was so much confusion about CTMS, I dug up the following slide that I use to help differentiate the various eClinical technologies and their role in clinical trials:

Fortunately, I was able to explain what was going on and helped them identify which eClinical systems were the source of data in their various reports. However, this experience got me thinking about the duplication of clinical data that exists in the different eClinical systems and how these ‘integrated suites’ of eClinical systems have evolved, and it led me to the question, ‘Is CTMS dead?’
Alarming Statistics & Trends:
My initial thought was, how could CTMS be dead? Afterall, CTMS was the original eClinical system and is the foundation for all other eClinical tools, right?
Upon typing ‘CTMS’ into Google, I was relieved to see pages and pages of results, most of which were different vendors offering CTMS, so certainly CTMS must not be dead. However, I also found some alarming statistics about CTMS usage. According to Tufts CSDD 2018 Impact Report summarizing the findings from the eClinical Landscape Study1, sponsors and CROs use an average of six applications to support clinical trial activities and over the past five years, CTMS has dropped to 5th place, behind EDC, IxRS, eTMF, Safety systems. Another survey conducted by Center Watch in 20192, also confirmed this trend and found that 93% of respondents said they use a standalone EDC system, 77% use an eTMF system, 71% a randomization and trial supply management (RTSM) system, and 70% still a clinical trial management system (CTMS).
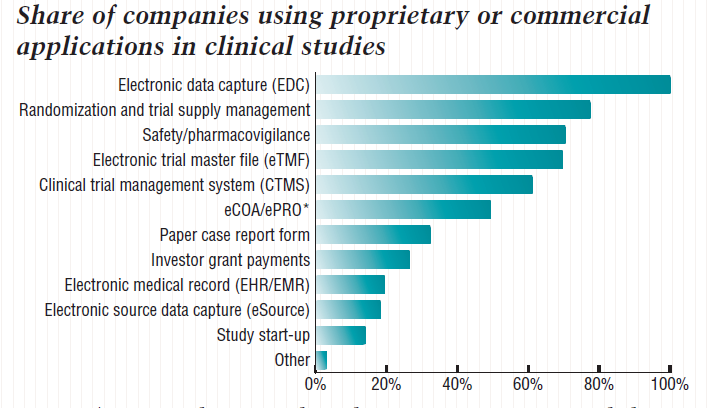
I was relieved to see that nearly 70% of companies use CTMS. Clearly, CTMS is not dead but if CTMS usage is dropping relative to these other systems, I started to wonder if CTMS was slowly being replaced. After all, there is a lot of overlapping clinical data in all the various eClinical systems used today and more and more specialized eClinical systems are getting introduced to the industry each year.
Certainly, real-time enrollment data can be gotten from the Randomization and Trial Supply Management (RTSM) system or even EDC, which is used in most every clinical trial these days. In fact, a lot of the clinical data that was traditionally ‘captured’ in CTMS during the days of paper trials is now being directly captured in the other eClinical systems that have become commonplace in clinical trials. In addition to enrollment data, RTSM systems contain drug supply information, which is also tracked in some CTMS systems. eTMFs not only store the study documents but also contain key dates, such as when the document was sent or received, as well as workflows that could automatically trigger the Site Activation process – just like many CTMS offerings. EDC is the most widely used eClinical system and contains even more data that overlaps with CTMS data.
EDC systems also contain enrollment data, as well as many other study status metrics, such as retention metrics, protocol deviations, open & closed queries, resolution times, etc. All this data is duplicated in CTMS and used to assess the progress of the study and site performance. In fact, much of this data is used by monitors during site visits and remote monitoring, so one could argue that site monitoring could be done in the EDC, rather than the CTMS. EDC systems are also preprogrammed with visit schedules and since the respective data is entered directly in the EDC, so it would be a more accurate source of information used to initiate site payments. And of course, EDC also has a complete listing of all sites, their addresses, contact information, and even investigator performance metrics can be determined directly from the system.
These are just a few examples from just a few eClinical systems. Given the increasing number of systems and the increasing overlap of data, one could easily assume that CTMS software is becoming obsolete. But if that were the case, why would there be so many CTMS offerings, perhaps more than ever before?
Today’s CTMS Landscape
Thinking about confusion my client had experienced, I decided to take a closer look at some of the CTMS offerings that are out on the market today. I first did a search on ‘Top CTMS’ and ‘Best CTMS’ and immediately noticed that they included a mix of CTMS, EDC and eTMF. In one list, they included SmartSheets, which is a timeline management tool, like Microsoft Project. Upon closer inspection, I noticed that many of these lists were Clinical Trial Management Software, not ‘System’. Therefore, I quickly realized that a large part of people’s confusion about, ‘What is a CTMS?’, is because the acronym can be misleading and vendors are using it to push their offerings, even if they are not what we consider to be a traditional CTMS.
After weeding out all the Clinical Trial Management Software vendors, there was still a large number offering Clinical Trial Management Systems, so I took a deeper look at the features and functionality of the various systems. Each vendor’s CTMS offering was slightly different. Some stored documents and facilitated document collaboration, like an eTMF; some had site payment modules, but others did not; and one had a patient database and facilitated patient recruitment. In most cases, it was hard to tell if their ‘CTMS’ offering was truly a stand-alone system or if their suite of eClinical tools together contained the functionality of a CTMS.
The one-stop-shop model isn’t a new concept. Early in the eClinical sub-industry, vendors had tried this approach but very few were successful. There were three reasons this model didn’t work: 1) The pharmaceutical industry just wasn’t ready yet (at that time, EDC was still only used in approximately 30% of clinical trials); 2) sponsors didn’t want to be tied to one vendor/system or forced to change out multiple systems at once; and 3) most vendors started out by specializing in one technology, then added other eClinical systems to their integrated suite but the other offerings were not as good as their flagship system. Today, this model may have a better shot of success, given how widely used eClinical system are today. However, vendors still need to make sure that every one of their offerings is the best in class (or at least a close second).
10 Reason Why a CTMS is Still Needed
Despite the growth of the eClinical subindustry and the increased outsourcing of clinical trials, below are 10 reasons why a CTMS is still necessary – and may in fact be the most important system for sponsors to have in-house:
- Single, Centralized, Source of the Truth: Today, pharmaceutical companies outsource approximately 60% of their clinical trials to CROs. Almost every CRO has its own set of eClinical tools (including CTMS) and very few are entering data into all the various client eClinical systems. Therefore, a company’s clinical data is spread over many different systems (and different companies), so a CTMS is still necessary to have a consolidated view of your portfolio, which enables companies to prioritize, manage resources, and leverage historical data to improve processes and efficiency.
- Reporting & Analytics: Without having all your data in one place, it is very difficult to produce comprehensive reports and perform meaningful analytics; not to mention the fact that out-of-the-box reports of most eClinical system don’t satisfy senior management’s expectations. Having a CTMS with a robust reporting engine (or the ability to easily connect to one) is essential these days and may be one of the most critical pieces of functionality because, this is how senior management interacts with the CTMS.
- Investigator Database: Most all CTMSs have an investigator database, which can be built up over time and used to help study teams select the best investigators for their trial. According to Tuft’s, 37% of site under-enroll and 11% fail to enroll a single patient3 throughout the entire trial. This is a huge inefficiency that costs companies millions of dollars each year but it’s one that can be easily be avoided. Many sponsors reply on their CROs to find and manage investigators, and they don’t have a historical record about their interactions or the investigator’s performance, which is critical to avoid costly, unfruitful engagements.
- Site Surveys & Communications: This goes hand-in-hand with an investigator database but having a system that enables sponsors to survey or communicate with large numbers of investigators and site personnel is a key part of site engagement. This is sometimes called Investigator Relationship Management (IRM) or Site Relationship Management (SRM). While there are plenty of survey tools, many sponsors are burdening sites with unnecessary surveys and questionnaires. Often sites are asked to provide the same information repeatedly to each study team and sponsor, when most systems will retain previous responses to standardized questions, reducing the burden on the site staff having to answer the same questions repeatedly. Additionally, now that electronic communications are becoming the norm in clinical trials, many modern CTMSs have some type of portal or ability to track all communications with the site. Not only does this further reduce the burden on the site but also can increase compliance by providing evidence of when communications were sent, received, and even read.
- Monitoring: Despite the increased presence of remote and risk-based monitoring functionality in EDC systems, the CTMS monitoring module is far from obsolete. Sure, EDCs could be built out to facilitate all of the non-data related monitoring activities, but first and foremost it’s bad practice and a regulatory risk to use a system that collects clinical data, for anything but that. Secondly, monitoring of sites requires a lot of flexibility and functionality that would be costly to pay an EDC vendor to configure (and validate) for every study. Even if sponsors set up their studies in an EDC system themselves, site monitoring still involves a lot of ‘paper’ trip reports, so the system must have the flexibility to handle and adapt to each sponsor’s business process.
- Project Management – Running a clinical trial is like managing any other project. It requires a good clinical project manager and a good project management approach. Modern CTMS systems are more than just a database used to store information. Many provide the tools and functionality to help streamline and improve the clinical study management process. Many of today’s CTMS systems enable study managers to build project plans (or sync with Microsoft Project), capture and manage Issues, Risks, & Action Items, schedule and assign tasks, customize business rules and processes, etc. Not only do these tools help guide study teams through the company-specific business process, but they also make it easy for clinical study managers to share and communicate this information with all the stakeholders.
- Milestone Tracking: While some study teams use timeline management software, like Microsoft Project, to create project plans for their studies, most clinical study managers either do not have the software, know how to use it, or just don’t want to use it. Even if they do, often the project plans lack the detail needed to keep track of all the various activities that must be completed at the study, country, and site levels. A good CTMS will have extensive lists of study, country, and site-specific milestones that automatically get applied to every entity and serve as a checklists, making it easy for large study teams to coordinate events, capture planned & actual dates, and keep others in the workstream updated on the status of the various entities and activities.
- Budgeting & Tracking: Similar to clinical milestones, budgeting and tracking of all activities at the various study levels involving numerous entities, can quickly become tedious. Again, a CTMS provides all the study-specific tools and makes it easy for clinical study managers to keep their study on track and on budget. Also, by having all study budgets together, in a central system, the financial health of each study in a company’s pipeline can be easily assessed in a single view. Most importantly, a CTMS stores the study costs (and vendor fees) over time, making it easier to produce more accurate budget forecasts for upcoming studies.
- Resource Planning & Management: Whether you are a manager or field monitor, a CTMS makes it much easier to schedule and manage site visits. Managers can easily see which monitors are available and assign them to sites in their region. Monitors can easily view their calendar, drill down into the specifics of each site visit, and manage their workload. Most CTMS systems have workflows that automatically send notifications and help managers, monitors, and sites coordinate activities and communicate plans and changes. Lastly, using a CTMS to assign personnel to various studies and activities, enables them to leverage the CTMS data to facilitate enterprise resource planning and demand forecasting.
- Data Integration & Workflows: More and more, the CTMS is becoming an eClinical data hub. For years, pharmaceutical companies have built homegrown data warehouses to consolidate and normalize the data from multiple sources and systems. Today, that process is a lot easier, mainly due to the advances in cloud technology. Some CTMS systems have become ‘platforms’ that either contain other eClinical functionality or are able to connect to other eClinical systems. Perhaps the biggest advancement and most widely used feature is the ability to import and export data directly from/to Microsoft Excel. As mentioned above, sponsors’ study data is spread over multiple companies and clinical systems, so it would be costly to integrate every system, but enabling study teams to import data from Microsoft Excel spreadsheets or CSV files reduces the time, cost, and the burden of (duplicate) data entry. Additionally, advances in cloud technology have made it easy to configure workflows to support a company’s business process. Whether it’s routing documents, firing off a multi-step approval process, or triggering a site shipment, a CTMS can remove many of the inefficiencies associated with running a single clinical trial, let alone 50 or 200 trials a year.
Conclusion
A CTMS is not just a ‘catch all’ for the miscellaneous data that doesn’t get captured in other eClinical systems, rather it is the central node that ties all the other eClinical systems together, making it possible to see the big picture and make informed decisions. Small- to medium-sized pharma companies have low adoption rates when it comes to implementing CTMS because they feel the CROs will track everything, so why incur the cost? However, newer cloud-based CTMS systems are very cost effective and have much more utility than just storing data. All companies could benefit greatly if they look at CTMS as a clinical project management and business intelligence system, rather than as just another clinical data system. As additional, more specialized, eClinical systems continue to emerge, vendors must adapt and evolve their CTMS offerings to provide functionalities that will support the way study teams work in today’s heavily outsourced environment and address the inefficiencies on the management and operational sides of research. CTMS isn’t dead after all – it has just transformed into something better.
References:
1 “Clinical Data Volume and Diversity Pose Increasing Challenges and Delays”; Tufts Center for the Study of Drug Development (CSDD); Impact Report; Jan/Feb, Vol. 20 No. 1
2 “CROs Moving to Electronics, But Paper Still Prevalent, Survey Shows”, CenterWatch Online, November 2019. Available: https://www.centerwatch.com/articles/24440-cros-moving-to-electronics-but-paper-still-prevalent-survey-shows
3 “Patient Recruitment and Enrollment in Clinical Trials”, Forte Research, October 2019. Available: https://forteresearch.com/news/infographic/patient-recruitment-enrollment-clinical-trials-infographic/